CO2 Analysis
Why do we care about CO2 in the ocean?
The carbon cycle in the ocean is complex, and is influenced by biological, physical, and chemical processes. In the ocean’s surface, CO2 is taken up by phytoplankton during photosynthesis. At depth, respiration produces relatively high CO2 concentrations, which can be brought to the surface by vertical mixing. For reference, sea surface p(CO2) in the absence of these physical and biological processes should be in equilibrium with the atmosphere at about 385 μatm. Phytoplankton blooms such as the red tides we have become accustomed to seeing in Monterey Bay can draw sea surface p(CO2) way down, as low as 150-200 μatm. Conversely, coastal upwelling of deep water can bring very high p(CO2) water to the surface, perhaps 800 μtam during extreme springtime events. In either case, disequilibrium is created at the air-sea interface, and the ocean can either release CO2 to the atmosphere or take it up.
On long time scales, the ocean is a major sink for CO2. Much of what is emitted into the atmosphere ultimately ends up in the ocean through air-sea exchange. We know that increased CO2 in the ocean promotes ocean acidification, which can be detrimental to certain organisms. Other biological consequences, such as changes to the dynamics of phytoplankton blooms, may be more subtle.
The Instrument
The CO2 sensor currently deployed at the Santa Cruz Wharf was built by Atmospheric Observing Systems (www.aosinc.net). They specialize in atmospheric CO2 measurement, but began observations of the coastal ocean 5 years ago. This instrument has been used extensively on ships, mapping p(CO2) in the Monterey Bay region. It will remain at the Santa Cruz Wharf for the foreseeable future.
The system uses an optical cell where CO2 concentration is determined by absorption of infrared light. Rather than measuring the water directly, it is pumped through a small showerhead in an equilibrator. Air in the equilibrator mixes with the water until they have the same CO2 content, and the air is then analyzed.
Periodically, a set of reference gases with known CO2 are flushed through the analyzer for calibration and to make sure it accurately predicts a wide range of CO2 concentrations.
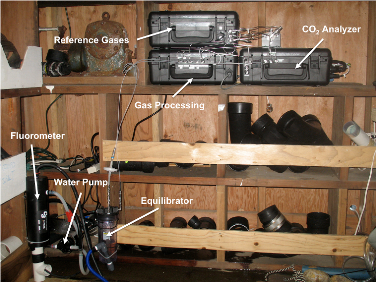
The p(CO2) instrument deployed at the Santa Cruz Wharf. Upper right are three cases housing components of the instrument. One holds reference gases for calibration and quality control of the sensor, another holds pumps and valves for gas processing, and the third holds the optics and electronics of the sensor itself. These are connected by tubing to an equilibrator (lower left), where seawater is pumped and mixed with the air to be sampled. Also shown is the Turner Designs C3, used to measure temperature, chlorophyll fluorescence, colored dissolved organic matter (CDOM), and turbidity.
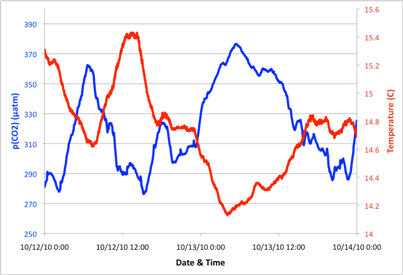
Sample data from 48 hours of observations at the Santa Cruz Wharf. Temperature and p(CO2) are clearly anti-correlated. During the day, the sea surface warms and p(CO2) is drawn down by photosynthesis. At night, biological activity drops off and p(CO2) increases due to exchange with the atmosphere or mixing with relatively high CO2 water nearby.
|



